Patient safety in medical research is of paramount importance, serving as the foundation for ethical and effective scientific inquiry. As institutions like Harvard face unprecedented funding cuts, particularly due to administrative mandates, the implications for patient protection are grave. The integrity of medical research oversight hinges on robust systems such as Institutional Review Boards (IRBs), which are tasked with safeguarding participant rights and ensuring ethical compliance. With diminished NIH research funding, the ability to conduct thorough reviews and improve clinical research ethics is compromised, thus raising concerns over the welfare of research participants. As the landscape of medical research shifts, ongoing dialogue about patient safety must remain at the forefront of discussions concerning funding and oversight.
The commitment to safeguarding individuals involved in health research holds vital significance for the entire medical community. Terminology such as human subject protection and clinical trial ethics reflects the same essential principles that underpin patient safety in medical research. Institutional oversight mechanisms like IRBs champion the rights and welfare of participants, ensuring that ethical standards are met throughout the research process. On the other hand, challenges posed by funding cuts to research, particularly from key bodies like the National Institutes of Health (NIH), threaten the effective functioning of these protective systems. As we advance in our understanding of medicine, the imperative to prioritize participant safety remains a crucial topic worthy of continuous attention and advocacy.
The Importance of Patient Safety in Medical Research
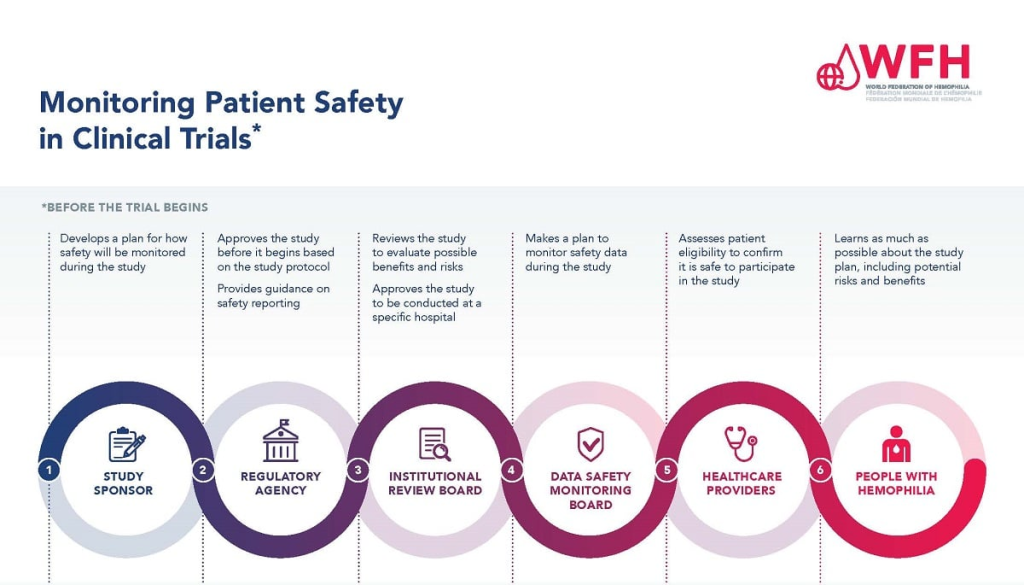
Patient safety is an essential foundation of medical research, ensuring that participants are protected from harm while scientists explore new treatments and therapies. Institutional Review Boards (IRBs) play a pivotal role in this aspect, carefully reviewing research proposals to evaluate the potential risks and benefits. Their oversight includes assessing the validity of the research question, the ethical treatment of participants, and the adequacy of the informed consent process. By focusing on patient safety, researchers can conduct their studies within a framework that prioritizes the well-being of all individuals involved, reinforcing public trust in the research community.
Moreover, the emphasis on patient safety is not just a regulatory requirement but a moral imperative in the conduct of medical research. The turbulent history of unethical medical experiments has led to stringent regulations to safeguard participants. By mandating comprehensive oversight, the research community actively seeks to prevent any potential violations of ethical guidelines, such as those exemplified in historical scandals. This ongoing commitment ensures that advancements in medical science do not come at the expense of participant welfare, aligning research goals with the highest ethical standards.
Impact of Funding Cuts on Medical Research Oversight
Funding cuts pose a significant threat to the oversight framework essential for maintaining patient safety in medical research. The recent cessation of over $2 billion in federal research grants has led to immediate and cascading effects on several ongoing studies. Institutions reliant on these funds face not only the disruption of existing research but also the potential halting of new projects crucial for advancing medical knowledge. Without adequate financial resources, the capacity of IRBs and other regulatory bodies to conduct thorough oversight diminishes, directly jeopardizing participant safety and the integrity of research outcomes.
As budgets tighten, the ability to adequately monitor and support clinical research becomes increasingly compromised. IRBs, which are essential for evaluating research proposals and protecting human subjects, often operate on grants and institutional funds. Reduced financial support leads to understaffing, affecting their thoroughness and efficacy in scrutinizing research projects. This can result in lapses in critical ethical oversight, diminishing the medical community’s commitment to protecting the rights and welfare of participants, ultimately undermining public confidence in future research endeavors.
The Role of Institutional Review Boards (IRBs) in Patient Protection
Institutional Review Boards (IRBs) are vital entities in preserving patient protection within medical research. These boards are tasked with the meticulous evaluation of research proposals, ensuring that appropriate measures are in place to safeguard participants’ rights and welfare. They assess recruitment methods, study protocols, potential risks, and the adequacy of informed consent processes—acting as a crucial buffer against potential ethical violations. The depth of scrutiny provided by IRBs enables researchers to conduct their studies responsibly and ethically.
Furthermore, IRBs contribute to the ongoing education and guidance of researchers, helping them navigate complex ethical issues that may arise during the research process. By providing training and resources, these boards foster a culture of compliance and ethical awareness among researchers. The collaborative nature of IRBs, which often includes community representatives, also ensures a broader perspective on the implications of research studies, reinforcing the commitment to participant safety and ethical standards in medical research.
Navigating Ethical Challenges in Medical Research
Ethical challenges are an inherent aspect of medical research, and navigating them requires careful consideration and vigilance. Historical events such as the Tuskegee Syphilis Study and the Willowbrook Hepatitis studies have illuminated the dire consequences of neglecting ethical standards in research. These past abuses have not only eroded public trust but also underscored the necessity of having rigorous oversight systems like IRBs in place. By examining potential ethical dilemmas early in the research design process, researchers can proactively address concerns, ensuring that participant welfare is not sacrificed for the sake of scientific advancement.
Moreover, ethical dilemmas in research often extend beyond individual studies, impacting entire fields of study and public health initiatives. Issues such as data privacy, informed consent, and the equitable treatment of vulnerable populations need ongoing attention and robust oversight. As research methodologies evolve, particularly with the rise of digital health technologies and genetic studies, IRBs must adapt to new ethical challenges. Their role in scrutinizing research frameworks ensures that evolving practices continue to uphold essential ethical principles, promoting the responsible conduct of research.
The Consequences of Insufficient Research Oversight
Insufficient research oversight can have profound consequences on both participant safety and the integrity of scientific findings. When regulations are undermined or compliance is neglected, the potential for harm to participants increases significantly. This can lead to adverse events, unethical treatment of subjects, and ultimately, public disillusionment with the research community. Such outcomes can hinder progress in medical science, as trust is a critical component of clinical research; without it, recruitment for future studies may dwindle, stalling innovations that could benefit society.
In addition, the lack of proper oversight may result in incomplete or biased data, further compromising the validity of research outcomes. If scientists are unable to conduct studies with sufficient ethical scrutiny, the resulting findings could mislead both practitioners and policymakers, affecting healthcare practices and public health strategies. Thus, reinforcing oversight mechanisms through adequate funding and support is essential not only for the protection of participants but also for ensuring the reliability and credibility of the research being conducted.
Training and Support Initiatives for Researchers
Training and support initiatives for researchers play a crucial role in fostering an ethical research environment. Institutions often provide workshops, seminars, and resources that equip researchers with the knowledge and skills needed to navigate the complex ethical landscape of medical research. These educational programs include topics such as informed consent, the importance of participant confidentiality, and the responsibilities associated with human subject research. By prioritizing these training sessions, institutions help cultivate a culture of accountability and ethical compliance within the research community.
Moreover, ongoing support from IRBs and ethical review boards is essential in guiding researchers through the compliance processes. As research regulations evolve, continuous education ensures that researchers remain updated on best practices and legal requirements. This proactive approach not only safeguards participant safety but also enhances the overall quality of research outputs. By effectively supporting researchers, institutions can mitigate risks and foster an environment of mutual respect and ethical behavior across all stages of the research process.
Community Engagement in Medical Research Oversight
Community engagement serves as a crucial element in the oversight of medical research, promoting transparency and trust between researchers and the public. Engaging with community members helps researchers understand the perspectives and concerns of potential study participants, which can shape research design and recruitment strategies. By incorporating community feedback, researchers can ensure that their studies are relevant, respectful, and ethically sound, addressing the specific needs and risks associated with the population involved.
Additionally, community engagement initiatives can empower participants, enhancing their understanding of research processes and their rights as subjects. When researchers actively involve community representatives in the oversight process, it fosters a sense of ownership and participation among individuals who may be impacted by the findings. This collective effort towards ethical research not only strengthens participant safety but also builds public trust in medical research initiatives, encouraging greater involvement and support from the communities served.
The Future of Clinical Research Ethics in an Evolving Landscape
As the landscape of clinical research continues to evolve with advancements such as artificial intelligence (AI) and big data, the ethical considerations surrounding research practices must adapt accordingly. Emerging technologies present new opportunities for innovation but also raise complex ethical questions regarding data privacy, informed consent, and participant autonomy. Therefore, it is imperative that ethical oversight, particularly through IRBs, evolves in tandem with these advancements to ensure that participant safety remains paramount.
Looking into the future, the integration of community perspectives in shaping ethical guidelines will be essential. As new research methodologies develop, ongoing dialogue with stakeholders, including participants, advocates, and regulators, will help to identify emerging ethical challenges. This inclusive approach will not only enhance compliance with research ethics but also cultivate a research environment rooted in trust and collaboration. Addressing these evolving ethical challenges proactively ensures that the benefits of research remain accessible and equitable for all.
Frequently Asked Questions
How does medical research oversight ensure patient safety in clinical trials?
Medical research oversight is critical in safeguarding patient safety during clinical trials. It involves independent review by Institutional Review Boards (IRBs), which assess research protocols, evaluate participant risks, and ensure informed consent processes are thorough. This oversight guarantees compliance with ethical standards and federal regulations, thereby protecting the rights and welfare of patients involved in medical research.
What role do IRBs play in protecting patients involved in medical research?
IRBs are essential for ensuring patient safety in medical research. They meticulously review study designs, assess risk levels, and monitor ongoing research for patient safety compliance. Their evaluations help prevent potential harms to participants and uphold ethical standards, making them integral to protecting patients in all phases of clinical research.
How do funding cuts impact patient safety in medical research?
Funding cuts can severely compromise patient safety in medical research by disrupting IRB operations and halting ongoing research projects. When funding is limited, institutions may struggle to maintain compliance with safety protocols and ethical standards, potentially leading to insufficient oversight and increased risks for participants in clinical trials.
Why is NIH research funding important for patient protection in medical studies?
NIH research funding is crucial for patient protection as it supports the necessary oversight and ethical review processes mandated by IRBs. These funds enable rigorous evaluation of research proposals, assuring that patient safety is prioritized and that the rights of research participants are upheld throughout the study lifecycle.
What ethical challenges does medical research face without adequate oversight?
Without adequate oversight, medical research risks ethical violations that can endanger patient safety. Historical abuses have shown that lack of IRB oversight may result in unintended harm, breach of informed consent, and erosion of public trust in clinical trials. Ensuring robust medical research oversight is essential to uphold ethical standards and protect patients.
How does informed consent contribute to patient safety in medical research?
Informed consent is a key aspect of patient safety in medical research. It ensures that participants are fully aware of the research scope, risks, and potential benefits before agreeing to partake in a study. This transparency empowers patients to make informed decisions, thereby enhancing their protection and well-being throughout the research process.
What historical events led to the establishment of medical research oversight systems?
Historical events, such as the Tuskegee Syphilis Study and unethical experiments during World War II, highlighted the need for stringent medical research oversight. These incidents prompted the establishment of IRBs and federal regulations to safeguard human subjects in research, ensuring that patient protection remains a top priority in medical studies.
Why are collaborative research efforts important for patient safety in medical trials?
Collaborative research efforts, supported by systems like SMART IRB, enhance patient safety by allowing multiple institutions to work together efficiently while maintaining regulatory compliance. This collaboration leads to improved data sharing, standardized protocols, and faster innovations that ultimately benefit patient care and safety in medical research.
| Key Points | Details |
|---|---|
| Funding Disruptions | The Trump administration’s freeze of over $2 billion in federal research grants to Harvard disrupts patient safety efforts in medical research. |
| SMART IRB Role | SMART IRB helps coordinate oversight of medical research across multiple sites to ensure patient rights and safety. |
| Importance of IRBs | Institutional Review Boards (IRBs) scrutinize research proposals and manage participant safety through review processes. |
| Potential Risks | Funding cuts may hinder research efforts and public trust, potentially harming the health and safety of study participants. |
| Historical Context | Past unethical research practices have led to the establishment of IRBs to protect research subjects. |
| Ongoing Collaboration | Despite funding issues, Harvard Medical School continues to support collaborative research efforts essential for public health. |
Summary
Patient safety in medical research is paramount. The recent funding disruptions have profound implications for ongoing research efforts intended to protect the rights and safety of patients. The reduction in NIH funding jeopardizes the vital role of Institutional Review Boards, which are essential for ethical oversight and safeguarding participants in clinical studies. To ensure public trust and uphold safety standards, it is crucial to address these funding challenges and maintain robust collaborative frameworks like SMART IRB.